Abstract
Acute myeloid leukemia (AML) is one of the extreme outcomes of age-related clonal hematopoiesis (ARCH). In recent years, data on differences between pre-AML and ARCH have emerged. However, it is still enigmatic why ARCH is prevalent in the general aging population, whereas only very few individuals eventually develop AML. Whether ARCH is solely related to abnormal hematopoiesis or originates from impaired microenvironment (ME) needs to be elucidated. The current study aimed to explore dynamics of molecular changes occurring in rare patients developing late post-transplant donor cell leukemia (DCL) and the relation of these aberrations to ARCH and/or ME.
We herein present two DCL cases, where the molecular profile was evaluated using deep error corrected sequencing (coverage ~4000X). In the first case, a female patient (26 y.o.) was diagnosed with AML secondary to myelodysplastic syndrome (MDS) and transplanted from her haplo-matched father (73 y.o.). Mutations at primary AML diagnosis were U2AF1 and TP53. Seventeen years later she devolved high-risk MDS, carrying PPM1D and EZH2 mutations. At the time of the DCL-MDS the patient had full donor chimerism and 100% XY donor cells by cytogenetics; however, both mutations could not be detected in the donor peripheral blood (PB). In the second case: a male patient (58 y.o.) with AML was transplanted in complete remission from his matched sister (52 y.o.). Primary AML mutations at diagnosis were U2AF1 S34F and RUNX1. Nine years later a second AML evolved, carrying new mutations, including U2AF1 (S34Y instead of original S34F), DNMT3a and IDH1. At the time of DCL-AML the patient had full chimerism and 100% XX donor cells by cytogenetics. Remarkably, none of these mutations could be detected in the donor PB or germ line either at the time of presentation or years later.
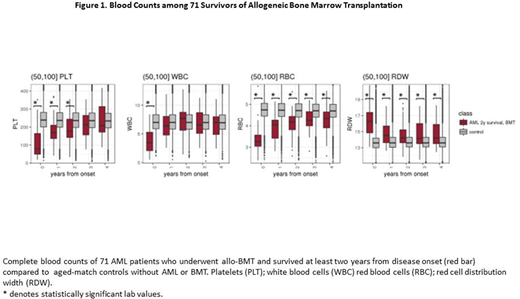
The chances of the same patient to develop AML twice in the same position (U2AF1 S34) or in the same pathway (TP53 and PPM1D) are less than 1:500,0002. While inherited predisposition to AML can explain such cluster of AMLs in the same patient, it is less likely to occur in the current cases, as no other leukemia has been reported among other family members. To further investigate the contribution of ME to leukemogenesis, longitudinal complete blood counts (CBC) were analyzed. While most of the CBC normalized in the years after the allogeneic bone marrow transplantation (allo-BMT), in both cases red blood cell distribution width (RDW) remained elevated, which could suggest underlying MDS. To explore this RDW pattern, we have followed various CBC indexes in a cohort of 71 allo-BMT survivors, from the dataset of the Clalit Healthcare Provider. Four years after allo-BMT, RDW was found to be significantly elevated, while red blood cell counts were significantly lower compared to those of age-matched controls. Other CBC parameters normalized 2-3 years post-transplant (Figure 1). Overall, these results suggest that at least in some AML cases even after allo-BMT, hematopoiesis cannot be fully normalized and the main manifestations are in the red cell lineage. Our unique DCL cases imply that the patient ME is involved in the abnormal hematopoiesis (increased RDW) and in the tendency of evolving a second AML, which has not developed in the donors.
Zuckerman:Cellect Biotherapeutics Ltd: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal